

Contact: Manager Yang
Hotline: 950-4048-3964 (free)
Tel: 0510-85386636
Mobile: 18011518665
Shangmeng Technology Wuxi Co., Ltd.
Address: A1-602, Tianan Smart City, No. 228 Linghu Avenue, Xinwu District, Wuxi City, Jiangsu Province
The silver cluster trapped in the zeolite is a porous material having small channels and voids and has remarkable luminescent properties. They can be used in more efficient lighting applications as an alternative to LED and TL lamps. Until recently, scientists did not know exactly how and why these small particles glowed. The team of interdisciplinary physicists and chemists led by KU Leuven is now demonstrating for the first time the origins of these properties.
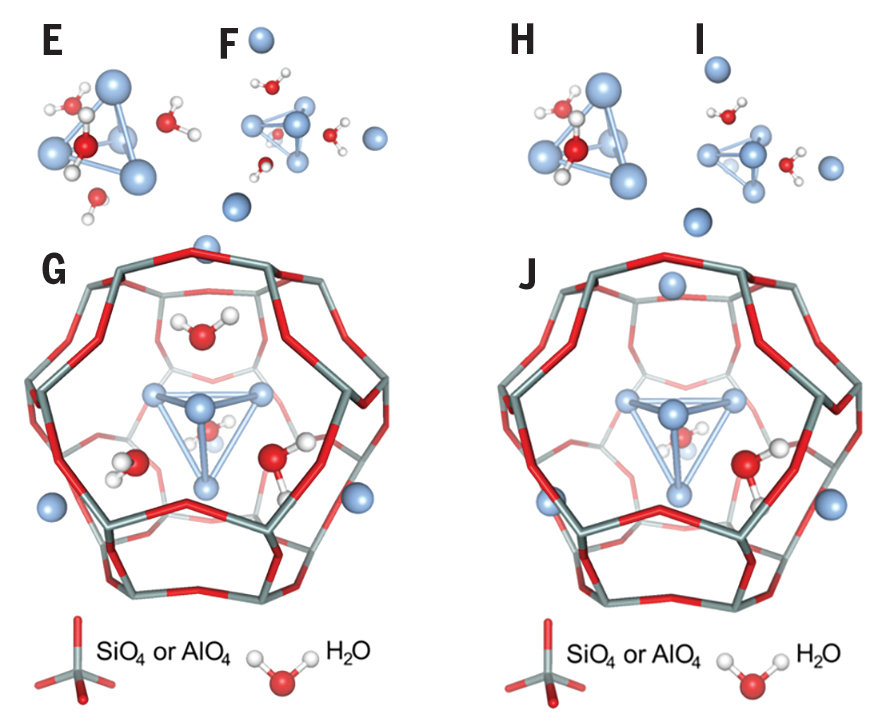
Zeolites have a very hard and well known structure and contain many small channels and voids. In chemistry, they are used to stimulate certain reactions. Molecules that "capture" in the pores of the zeolite lose their fluidity and begin to behave differently. This study enabled scientists to discover why and how luminescent clusters glow in cages of certain types of zeolites .
“We irradiated the mixture of silver clusters with synchrotron radiation in the European synchrotron radiation device in Grenoble,” researcher Didier Grandjean explained. “What good is this, it gives us a lot of information about the structure and properties of the material. However, because we especially want to see the optical properties, we have adopted a new method, deliberately only measuring the emitted light, we are sure that we only pay attention to Responsible for specific particles of light. " This study provides conclusive evidence that only four clusters of four silver atoms in the form of silver atoms are surrounded by water molecules and emit light.
"Tetrahedrons form a unit in which two electrons move freely. This forms a so-called super atom: a structure made up of several atoms, but behaves very much like an atom," said Professor Peter Lievens. "The optical properties of clusters are caused by two free electrons. They decay from higher energy levels to lower energy levels, resulting in a certain amount of green light. In turn, energy levels are determined by superchemical properties. Atomic." Observations can be attributed to close collaboration between researchers in chemistry and physics. “In addition, our experimental observations have been confirmed by advanced theoretical calculations,” said Peter Lievens.
This newly discovered knowledge enables researchers to begin modifying the properties of silver clusters or finding new materials with the desired optical properties . The results of this work are very important for studying light and its applications.
The study was published in the journal Science .
![]() Further exploration: scientists use silver to make the light bright
Further exploration: scientists use silver to make the light bright
More information: "The origin of the bright photoluminescence of a few atomic silver clusters confined in LTA zeolites" Science (2018). Science.sciencemag.org/cgi/doi ... 1126 / science.aaq1308
Address:Tianan Smart City A1-602, No. 228 Linghu Avenue, Xinwu District, Wuxi, Jiangsu, China TelePhone:0510-85386636 Fax:0510-85384339 E-mail:info@solmontech.com
KeyWord: